SCRC Partner Profile: A Textbook Transformation at Biogen-Idec
Although strategic sourcing capabilities are well established in industries ranging from automotive to electronics, they are still in the formative stages in the biotechnology sector. It isn’t easy to drive change in biotech companies for a number of reasons. With their external spend increasing so fast, there is great pressure on biotech companies to rethink how their sourcing structures are set up to add most value. In addition, much of the power in decision-making lies in the hands of clinical trials scientists, who often have power over procurement decisions. In a growing number of companies today, supply management is being asked to demonstrate deep insights into customer requirements, and to quickly translate those insights into product offerings that often rely more on outsourced capabilities.
Biogen Idec Inc. is showing how this can happen in the biotech sector. In the last several years, the company has undergone a huge shift in the way it operates its supply chain, particularly in the management of sourcing.
Headquartered in Weston, Mass. and with international headquarters in Zug, Switzerland, Biogen Idec is the world’s oldest independent biotech and a Fortune 500 company with more than $4 billion in annual revenues. The company discovers, develops, manufactures, and markets biological products for treating conditions such as multiple sclerosis and non-Hodgkin’s lymphoma. It was formed from the November 2003 merger of Biogen, Inc. and IDEC Pharmaceuticals Corp.
In addition to its portfolio of drug candidates, the company has capabilities, including capacity for protein manufacturing, that are world-class in quality and scale. Biogen Idec is one of a handful of biotechs that have licensed and dedicated biological bulk-manufacturing facilities. It has a large-scale manufacturing plant in Research Triangle Park, North Carolina—one of the world’s largest cell culture facilities—and a new 90,000-liter facility for producing biologics in Hillerod, Denmark.
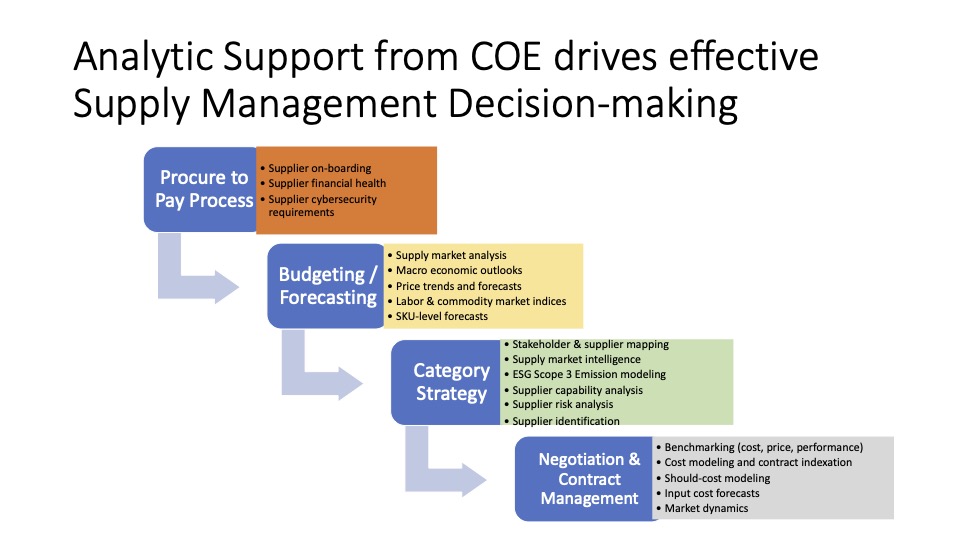
The company’s global operations (supply chain) unit reports into its pharmaceutical operations and technology (PO&T) business area. The global operations mission is to ensure the uninterrupted supply of the highest-quality products to patient sites worldwide. To meet its goals, PO&T sourcesan array of products and services, including active pharmaceutical ingredients, outsourced contract services, professional services, manufacturing and facilities equipment, manufacturing contract services, and raw materials and lab consumables. Like many other companies in this sector, external spend is significant in the range of several hundred million dolalrs annually, almost all of which can be leveraged.
Prior to 2009, support for PO&T sourcing and procurement was provided by a corporate sourcing group under a centralized business model. In September 2009, anticipating a sharp rise in the complexity of the company’s supply chain, the management team launched an assessment of supply chain maturity across PO&T. Several factors had converged to spark the maturity assessment. To begin with, there were many more clinical trials in the pipeline, dealing with a range of new technologies that had not been sourced before. Concurrently, contract manufacturing spend was increasing; As a result, the executives anticipated that workload would increase in multiple PO&T areas.
Category managers were mandated to bring new sourcing efforts to the council for approval, sponsorship and commitment. For example, the council approves new supplier recommendations, non-budgeted spend with non-approved suppliers, and major strategy changes with regard to supply chain configuration. In addition, the facilitator of the council meeting—operations analytics director—is tasked with presenting the monthly KPIs on the health of PO&T suppliers as well as the spend trends in order to ensure adherence to overall corporate goals. By institutionalizing robust supplier approvals and/or changes, there was far less chance that suppliers would be selected based on the priorities or wishes of individual departments as opposed to organization-wide imperatives. This framework also enabled a uniform, data-driven basis for selecting which projects to pursue, and to commit them to completion.
What began as a “grass-roots” transformation effort at Biogen Idec produced strong early results in the form of cost savings. The initiative was soon recognized by the executive team, but as the effort expanded, it produced results beyond cost savings, sustaining momentum and moving the organization to higher levels of process performance. This was achieved by focus on stakeholder engagement and use of an analytical sourcing framework. As a result of the partnership created between procurement and the R&D group, Biogen’s sales have risen as new products have been approved by the FDA, sending their share price soaring.
Sources: Ganguly, Joydeep, Shepherd, Alasdair, Alegria, Esther, Ciamarra, Rob, and Handfield, Robert, “A Textbook Transformation: How Biogen Idec Overhauled its Supply Chain”, Supply Chain Management Review, May/June, 2011, pp. 28-35.