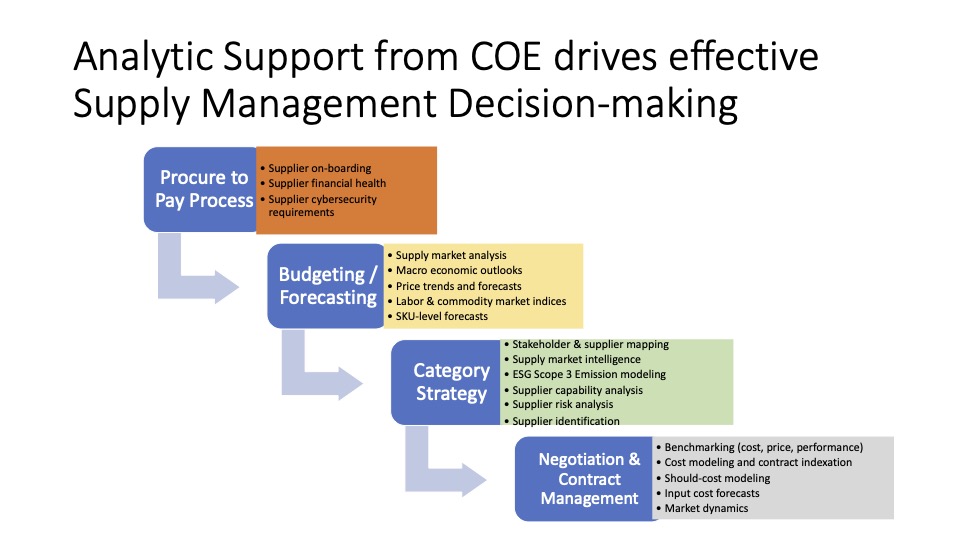
“One Word – Are you Listening? – Plastics”: A Critical Bottleneck to COVID Testing


This famous line from “The Graduate” describes one of the major bottlenecks that exists in the PCR testing supply chain, as we learned in our research interviews this week. I am working with a team of faculty and students on the COVID-19 testing supply chain, and we are seeking to try to develop the most effective and efficient testing strategy that will be the most effective for the state and for the country.
One of the problems we learned on the supply side of the test market is a function of the number of machines available for processing the PCR molecular testing, which are considered the gold standard for COVID testing. PCR molecular nucleic tests are the most accurate, but also the most expensive to run. Prior to COVID, the capacity of PCR test equipment manufacturers was perfectly sized to the amount of money insurance companies were willing to spend on reimbursement. Molecular testing is more expensive than amino and chemical testing and is inherently better. However, the insurance companies and healthcare systems didn’t support full utilization of molecular testing, as it is more expensive compared to older technologies. For that reason, the market capacity was perfectly sized for a normal situation when Roche would sell maybe 200 units a year.
That is, until COVID started spreading this spring, and the demand for PCR testing equipment went through the roof. Roche is currently scaling up to get to 1000 machines per year, and will hopefully reach this capacity by January. The Roche 8800 COVID machine can push out about 3000 tests per day, as it is highly automated. Tests are loaded into the machine, the technician can walk away, and come back later to collect the result from the sample, making it a “walkaway” solution. Other solutions are basically deconstructed Roche 8800 machines, where all the pieces in the Roche unit are broken apart into separate machines, and samples must be transferred between them by technicians, making the solution more manual.
In July testing spiked, and most labs had demand that was well over their capacity, and turnaround times were extended as there was more work than capacity. Testing volumes modulated in August, and were flat for most of September, but began to start ticking up during the last two weeks of September. Right now testing volume is starting to spike again. Certain states have more testing available than others (New York is strong, Texas is light), but even places with higher infections rates don’t always seem to respond with investment in more testing resources.
But there is one hitch – plastics! The use of molecular equipment is paced by the marketplace, but by far the biggest challenge consistently (from March until now) – is the availability of plastic components. In all of the testing solutions that are out there plastics are the biggest constraint. For example, the Roche extraction units utilizes a customized plastic cartridge that hold s96 patient samples for extraction. These plastic cartridges are on worldwide allocation and Roche is struggling to get additional precision molds and suppliers to support their machine capacity. Another plastic component that is challenging are tips, which are used on automated liquid handling, and are on worldwide allocation. Finally, the plastic nose swabs used to collect PCR samples were all produced in a single plant in Italy in the Lombard area, (which was also shut down during the spread of the pandemic in this region back in March and April).
A good chunk of the plastic consumables capacity is coming out of SE Asia, and most small labs do not have direct sourcing relationships with the suppliers of these plastic components, and are thus on hold through their alternative distribution channels. Larger labs have engineering teams in Asia and are directly sourcing product, using procurement engineering team that are engaged in reverse engineering to find supply alternatives. Not surprisingly, purchasing engineering teams are generally not readily available in healthcare organizations.
A supply chan director at a mid-sized diagnostics lab noted the following: “Plastics suppliers are a big constraint. Once you commit to a machine, you don’t have a lot of ability to change suppliers or materials. And each machine has disposable materials that are all a little it different. There is only one type of plate that fits properly on the Hamilton machine, but because everyone wants it and doesn’t have the proprietary molds, everyone in the industry is on allocation and waiting. We have had an open purchase order since August, and our first delivery was supposed to be November, but it has been pushed back to Q1 of 2021. And we are prohibited from using another overseas manufacturer to make the plastic trays, since our domestic manufacturer of plastic trays cannot keep up with demand. This is because the trays are specific to Hamilton, and Hamilton has had a rule that they will not honor the warranty of their robots if someone buys consumables from somewhere other than the approved source. So if someone buys a plastic tray or a pipette tip from an unauthorized source, the warranty is automatically void. Yet Hamilton hasn’t been able to supply these components, and 10% of their orders are on allocation. So everyone is going outside the country to find these components during COVID. And for those of us who have invested millions of dollars with Hamilton, we can’t afford to use a machine where the warranty won’t be honored!”
The real solution here, however, is to begin thinking about the role that the easier, cheaper antigen test is going to play in combating this virus. PCR test are very accurate, and are generally good for diagnosing whether someone really as COVID. However, antigen testing can be used to screen people, especially students who are coming back to school or at universities. Abbott promised to deliver its first 150M tests to the US government, which are slowly being distributed this week. But this will requires states to also begin thinking about what their testing strategy should be. Our NC State team has developed what we think is a comprehensive testing strategy that employs both the antigen and the PCR tests. This, in combination with the vaccine deployment next spring, will hopefully create a light on the horizon.