Examining the Effect of Health Policies on the Opioid Epidemic from the Lens of Supply Chain
Guest post by: Amir Hossein Sadeghi and Amirreza Sahebi Fakhrabad
Opioid Epidemic in the U.S.
The opioid epidemic is a well-documented national crisis that has had a devastating impact on communities across the United States. In recent years, efforts to address the crisis have included state-level opioid prescription limits and the implementation of prescription drug monitoring programs (PDMPs). These interventions have been shown to reduce the over-prescription of opioids and prevent addiction. State-level opioid prescription limits are restrictions on the amount of opioid medication that can be prescribed to a patient. These limits are intended to reduce the availability of opioid medication for misuse and prevent the development of opioid addiction. PDMPs are government-run systems that track the prescribing and dispensing of certain medications, including controlled substances like opioid painkillers. These programs are used to monitor patients’ medication use and identify potential cases of prescription drug abuse or misuse.
Pharmaceutical Supply Chain
The opioid epidemic has been fueled in part by the actions of opioid manufacturers, who have been accused of misleading doctors and patients about the risks of these drugs and aggressively marketing them for uses that are not backed by scientific evidence. Some opioid manufacturers have faced legal action for their role in the crisis. However, few studies have evaluated the effect of state-level opioid regulations on the supplier side (distributors and manufacturers) as well as the consumers (patients and pharmacies). Figure 1 shows the key elements of the pharmaceutical supply chain, which refers to the series of steps and processes involved in the distribution and delivery of prescription and over-the-counter medications from manufacturers to patients. The pharmaceutical supply chain is a complex system that involves multiple stakeholders, including manufacturers, wholesalers, pharmacies, and regulatory agencies.
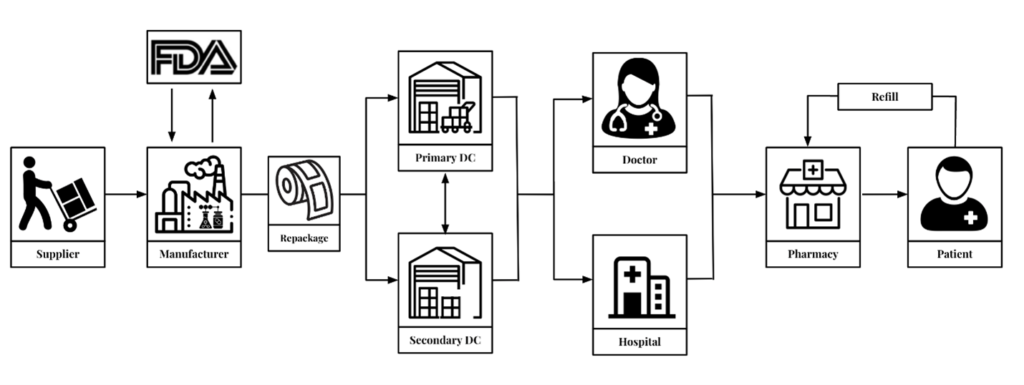
Efforts to address the opioid crisis should consider the role of all stakeholders in the pharmaceutical supply chain. This includes the need for greater accountability and transparency from opioid manufacturers and distributors, as well as increased access to addiction treatment for those who have become addicted to opioids. Ultimately, a comprehensive approach that addresses all aspects of the opioid crisis is needed to effectively address this public health concern.
The Power of Data Analysis and Machine Learning
The Drug Enforcement Administration (DEA) maintains a database called the Automation of Reports and Consolidation Orders System (ARCOS). This database records the authorized sales of controlled substances for both humans and animals in grams to medical facilities, pharmacies, and practitioners. A recent study by the expert research team of Supply Chain Resource Cooperative (SCRC) utilizes the ARCOS data to investigate the distribution of opioids across different states and identify potential policy implications [Hyper link:].
The team analyzed the ARCOS data from 2006 to 2014, which included information about the amounts of opioids sold and the parties involved in the transactions. The analysis focused on two opioid supply chain players: dispensers and distributors. The research analysis showed that policymakers should consider the potential for unintended distribution shifts of opioid drugs to neighboring states with laxer regulations as well as varying impacts on different dispenser types. It is crucial to identify potential sources of abuse or diversion of opioids, and policymakers must take appropriate measures to prevent the misuse and abuse of these drugs. The study highlights the importance of utilizing data-driven approaches to inform policy decisions and address the opioid epidemic in the United States.
Another study by the research group, advised by Prof. Rob Handfield and Eda Kemahlioglu-Ziya develops a classification system based on distance and disparity between dispensers, prescribers, and patients. This system grouped transactions into 16 categories based on the location of the stakeholders involved. The study examined the effect of the legislation on opioid prescription volume in each of these categories, as well as overall. The results showed that the policy was effective in reducing overall prescription volume, with a decrease from 53.68 daily MME before the policy to 51.09 daily MME after the policy. However, the study shows that the impact of the policy varied across the different categories. Specifically, we observed a smaller decrease in prescription volume in the categories where the prescribing doctor was located nearby, indicating potential leakage or circumvention of the policy in these cases.
To ensure the effectiveness of policies aimed at addressing the opioid epidemic, it is important to monitor and analyze their impact over time. This study demonstrates the potential usefulness of data analysis and machine learning in identifying areas of higher prescription volume and targeting policy interventions accordingly.
- Categories: