The COVID-19 Vaccine Supply Chain: Potential Problems and Bottlenecks
By Caroline Barnhill
As the first COVID-19 vaccines roll out across the country, pressure is mounting for these vaccines to be distributed and administered quickly. With hospitalizations increasing – and the United States lagging behind other countries in vaccinations – state and local policy makers are working to make vaccinations available for each segment of the population as soon as possible.
While Operation Warp Speed’s strategic plan assumes that vaccinations will go smoothly – at least after more doses become available – a deeper look at the global supply chain points to several potential issues down the road. In a webinar hosted on December 16 by the Supply Resource Chain Cooperative in the Poole College of Management at NC State University, our experts identify these possible hiccups – and urge key players to carefully consider these issues as they move forward with vaccine distribution and administration.
Meet the Experts
Dr. Robert Handfield, Poole College of Management
Dr. Robert Handfield is the Bank of America University Distinguished Professor of Supply Chain Management at North Carolina State University and the Executive Director of the Supply Chain Resource Cooperative. Additionally, Handfield serves as an adjunct professor with the Supply Chain Management Research Group at the Manchester Business School and as consulting editor of the Journal of Operations Management, one of the leading supply chain management journals in the field. He has consulted with more than 25 Fortune 500 companies, including GlaxoSmithKline, Freightliner and Boston Specific – and was identified as one of a handful of individuals in the 2007 “Pros to Know” list compiled by Supply and Demand Chain Executive journal. With extensive knowledge of supply chain and medical supply chain, Handfield is considered a thought leader in the field of supply chain management.
Dr. Daniel J. Finkenstadt, Naval Postgraduate School
U.S. Air Force Major Daniel J. Finkenstadt is an assistant professor in the Graduate School of Defense Management at the Naval Postgraduate School where he offers courses in enterprise sourcing, entrepreneurship and spend analysis. Additionally, Finkenstadt serves on the editorial board for the MDPI’s journal Logistics and NCMA’s Journal of Contract Management. He holds a Ph.D. in Marketing from the Kenan-Flagler Business School at the University of North Carolina at Chapel Hill. With 17 years of contracting experience in operational, systems center, headquarters, joint, overseas and classified environments, Finkenstadt brings a wealth of knowledge in government acquisition and enterprise sourcing for the federal government.
Handfield and Finkenstadt combine their expertise of the global supply chain, medical supply chain, government acquisition and enterprise sourcing for the federal government to evaluate Operation Warp Speed’s strategic plan and the national infrastructure available. From March until May of 2020, the two have worked extensively with the COVID-19 Joint Acquisition Task Force (JATF) and have collaborated with several agencies, including the Federal Emergency Management Agency, the U.S. Department of Homeland Security and the Strategic National Stockpile.
Below are some highlights from their discussion.*
*Please note some of the responses were condensed for clarity and brevity
Setting the Stage: A Fragile Medical Supply Chain
Handfield: The more we started to dig into the global supply chain and the vaccine, the more worried we got. While watching the news may make it seem like vaccinating the whole country is going to go smoothly, we still have several concerns about how this is all going to roll out.
First, it’s important to note that due to the nature of globalization, we’re living in a much different world. Many healthcare institutions have outsourced production of personal protective equipment (PPE) and healthcare supplies to suppliers in China. Since March, we’ve seen that a lot of these supply chains have essentially failed – posing real risks to the U.S. and other countries in Europe. Take large original equipment manufacturers like 3M, for instance – they have as many as 5,000 direct suppliers, and each of those suppliers have their own suppliers. This results in quite large supply chain networks that extend all over the world – and it only takes one incident to disrupt these operations. Plus, many organizations don’t even know who is in their supply chain. This is what we saw earlier on with N95 masks, gowns and gloves.
So what we have is a much more delicate or fragile supply chain for healthcare supplies, which really sets the stage for where we are now. Because the supply chain has become a much bigger factor, many of the components of the vaccine are subject to these same potential risks.
In Operation Warp Speed’s strategy for distributing the COVID vaccine, “From the Factory to the Frontlines,” the CDC explains that rolling out the COVID vaccine is going to be a three-phase project. The only bottleneck they anticipate will be in phase one for key healthcare workers and long-term care facilities. After this, they say, there will be plenty of doses available – at which point everyone will get vaccinated and COVID will go away. In other words, this plan assumes that everything is going to go smoothly.
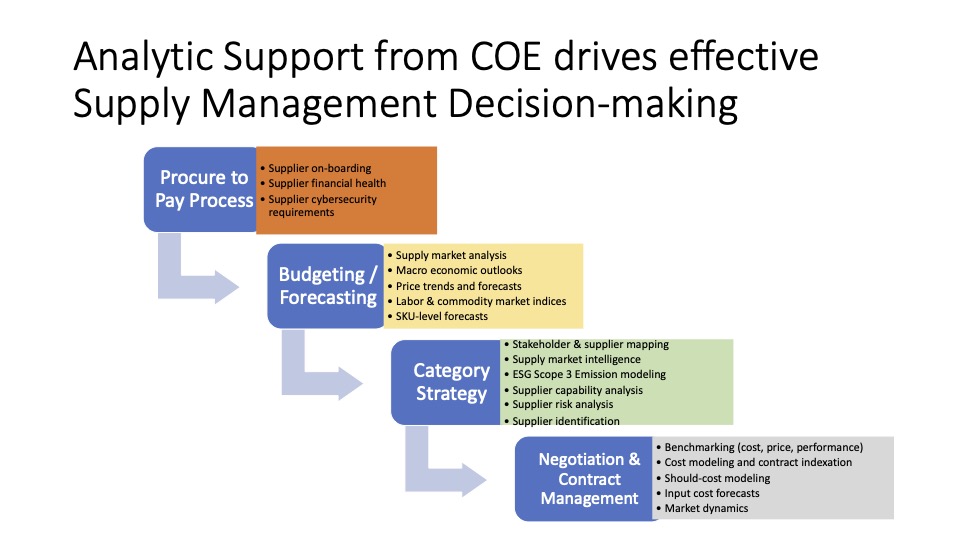
But as we started to dig in deeper, using a framework called the Supply Chain Operations Reference (SCOR) Model, we considered what bringing this vaccine out to the public really involves. And as we started looking at these different phases of the supply chain, we discovered that there are several potential hiccups and bottlenecks that may occur at every stage.
Potential Problems and Challenges
- National security issues
- Shortage of personnel
- Lack of coordination
- Shortage of supplies
- Limited capacity
- Vaccine damage
- Gaps for rural areas
- Misinformation about vaccines and tracking
National Security Issues
Handfield: This vaccine has been referred to as liquid gold. Anytime you have something that’s of high value, there’s potential for some level of nefarious activity to go on. There are a lot of security issues around the transfer points at each point in the vaccine supply chain. Potential risks include theft, sabotage and counterfeiting. For example, how do you dispose of the vaccines that are potentially damaged and the huge volume of healthcare waste being generated by vaccinations? The risk is that if these are not properly disposed of, they will end up in the hands of someone who’s going to market them on the black market or try to refill the containers with material that’s counterfeit.
Shortage of Personnel
Handfield: The first issue we found with Operation Warp Speed’s strategic plan is that it doesn’t account for vaccinating the vaccinators – there’s no plan in place for vaccinating the people actually administering the vaccine.
Finkenstadt: I have this visual of refueling aircraft. If you only worry about the F16 that’s flying around and don’t worry about who’s going to refuel the F16 to do the mission, then you just have a bunch of these F16s flying around with one tank of gas – and then they’re out. So as we think about the COVID vaccine, we have to vaccinate the entire value chain. This stems from the needle all the way back to who gets the vaccine in vaccinators’ hands – all the people along the way who make critical deliveries and logistical decisions to get the vaccine where it needs to be. If you don’t consider those folks, then you’re putting essential healthcare workers and the elderly at the front of the line without consideration of the fact that there are people who have to get the vaccine to them. These people should probably be in phase one as well.
We need to ensure that key supply chain personnel stay healthy. These include the thousands of people that all have critical roles – all the way down to the people who sanitize the plants. These personnel matter because if they’re not there vaccine manufacturing, distribution and administration will completely shut down.
We also need to consider the shortage of truckers in the United States – we have around 60,000 less truckers than needed. Even if production ramps up, we may not have the personnel needed to drive the deliveries where they need to go.
Lack of Coordination
Handfield: Though the strategic plan refers to collaboration on micro-plans at the local and state level, few details are provided – and it seems that effective planning has not yet been done. The communication between the states and the federal government seems spotty at best. Specifically, it’s unclear how the vaccines will be allocated on the ground – and how they’ll allocate who’s in the first, second and third waves of distribution. It’s also unclear whether people will be notified about the second dose of the vaccine – and whether they will be told which vaccine to get. But anyone who’s worked in supply chains recognizes that if you don’t have a plan to work, you can’t work the plan. And without a clear plan, there are several risks – potential shortages, hacking, IP loss, a lack of coordination that results in inequities, etc.
Finkenstadt: It sounds like the CDC plans to track vaccines distribution down to the hospital level, but the big hang-up here is that there will be multiple vaccines – and once an individual starts a vaccine series, they’re wedded to that series. Another thing to consider is that people may be physically locked down to a geographical area once they start a vaccine series, depending on how states determine the allocation. For example, if someone starts the Pfizer series, they need to know whether they can get their second Pfizer dose from any providing location – or whether they need to stay in their county, region or state. If that isn’t clearly communicated, it could lead to chaos. This all points to the need for tracking at the individual level, and perhaps we can think about ways to put that power into the hands of individuals through digital apps.
Handfield: Operation Warp Speed’s strategy document discusses constructing an IT infrastructure to monitor everything, but such a system does not yet exist. Plus, healthcare is notorious for having really poor quality data and data integration. So we’re concerned that this monitoring will be problematic moving forward.
Shortage of Supplies
Handfield: Though Pfizer has already manufactured 20 million or so doses, Pfizer, Moderna and other vaccines are experiencing severe bottlenecks due to a lack of critical materials – including vials and rubber stoppers for the vials. There’s also been a shortage of natural rubber, which is problematic because rubber gloves are recommended for administering the vaccine. If you lack access to these raw materials for the vaccines kits, you’re disrupted and scaling production comes to a grinding halt. While some suggest looking for alternative suppliers or substitute materials, that requires getting FDA approvals, which can take months in some cases.
Finkenstadt: This shortage on natural rubber is very concerning, especially because it’s not just COVID impacting it. Various factors, including environmental weather concerns, are impacting the sources for that. So as we look at supply chains and disruptions, we can’t forget that COVID is not the major disruptor day in and day out to these supply chains. Supply chain risks have always existed with things like weather patterns, factory shutdowns and factory fires. It’s just that these risks are more important than ever.
Limited Capacity
Handfield: On the production front, one of the biggest bottlenecks is what they call fill-finish capacity. This is the ability to take the liquid vaccine and put it into vials that can be distributed. Each vial carries a limited number of doses. Another limitation is freezer capacity. While the Pfizer vaccine needs to be stored in a freezer at -94 degrees, most freezers are not designed for that level of cold – most are designed for -20 degrees. Taking these factors together, there’s extremely limited capacity.
There are several risks here – equipment breaking down from being overrun, new equipment not meeting regulatory requirements, etc. Though we don’t often think about such things, these are all possible risks that could shut down the production process.
This also points again to a potential shortage of skilled personnel. It’s crucial that there are enough skilled personnel who are healthy and able to work in these fill-finish capacities and storage facilities. And, once again, are they being vaccinated so they can fulfill these roles?
Vaccine Damage
Handfield: There’s been a lot of media attention on delivery and cold chain – the ability to ensure that the vaccine remains at the proper temperature during its path from the manufacturing facility to a UPS or FedEx distribution center, then onto a truck with dry ice, and then to a state or local hospital.
Finkendstadt: Depending on the length of the route, dry ice packing has to be reaccomplished. Each time you open these packages up and try to recool them, you’re allowing for temperature excursions, which can potentially damage the vaccine inside. I think there’s a limit of two package repacks before, essentially, you can’t recover. This high-touch handling is very risky.
Handfield: What it really comes down to is that last mile. Once you take the vaccine out of the cold chain, it has a five-hour window, and then it starts to deteriorate. So there are some real concerns about that last mile and how to handle the vaccine being out of that cold chain.
If you’re in a major urban center, you’ll likely have access to the vaccine shot at your local hospital – they’ll have something set up for that. But if you’re in a rural area that doesn’t have a local hospital – or it has a very small hospital – some tricky issues may come up. How does the vaccine get to these smaller towns and cities, or to people who are in different facilities and don’t have access to transportation?
Here’s another example. I spoke with my physician and she asked, “What if they give me enough to do five vaccinations today, and five patients make an appointment to come in and get a vaccination – but only two of them don’t show up. What do I do with that leftover vaccine?” There needs to be a plan for handling no-shows to ensure no vaccine goes to waste.
We also need to consider malfunctions along the way – what if there’s a vehicle failure? What if there’s a power outage and the freezers no longer work? These are normal things that happen – and in each case, you’re dealing with this liquid gold that potentially could go to waste.
Finkenstadt: If equipment malfunctions, it’s important that we monitor inside the cold chain. We’re hoping that we can monitor these deliveries, at least at the pallet level, if not at the individual package level. At the pallet level, we might miss things and not know when we need to respond. It’s important to know if there’s a section of the vehicle where there might be a temperature excursion.
In urban areas with more resources, this may not be a major issue, but in rural and geographically separated areas, there is more opportunity for these supply chains to break down. It’s important to ensure that the cold chain and supply chain supporting freezers are able to prevent temperature excursions and prevent large amounts of this vaccine from spoiling.
Gaps for Rural Areas
Handfield: Using data from the Homeland Infrastructure Foundation, we identified what the public refrigerated warehouse map actually looks like. Major urban centers, especially the Northeast and Midwest, are in pretty good shape. But then you have this huge gap in the center of the country – the Dakotas, Montana, Wyoming, New Mexico, parts of West Texas – where there isn’t a lot of cold storage capacity.
Plus, most of this cold storage space only goes down to the negative 20 degrees or so – very little of it going down to negative 80 degrees. This creates a major gap in terms of the capacity and our ability to distribute it across the country. How do we create solutions for these locations? Remember, we need to get 60 to 70 percent of the country vaccinated and can’t leave out these rural communities.
Finkenstadt: We can’t forget to focus on the tribal communities and the reservations within the Americas, as well. However, these communities lack the ability to receive and distribute goods – which is really concerning. This makes distribution with cold chains particularly difficult.
Handfield: The best way to validate a supply chain is to trial it. So to handle these temperature-controlled environments in rural areas, run temperature excursions and tests at the pallet level with these containers – and track them with a GPS.
Misinformation about Vaccines and Tracking
Handfield: Early statistics show that as many as 60 percent of the population don’t want to take the vaccine. In minority communities, the number of those wanting to take the vaccine is as low as 10 percent. A lot of this stems from a mistrust of the government and misinformation about the vaccine.
There are also people who will think, “I got my first dose. I don’t need to go back for my second dose.” We need to educate people and make sure they understand the need to go back for that second dose – or else they run the risk of getting COVID.
Finkenstadt: People are also really concerned in this country with the idea of being tracked by the government, so this will play into how we communicate with them. How do we communicate that tracking through a personalized application is the safest bet for the whole country and for individuals – and that you’re not giving up your liberties or your privacy by using these?
Handfield: We need really smart healthcare personnel, legal minds and technical data experts in this space because they’ll have to balance full transparency into the chain of this vaccine without breaking any HIPAA rules or tapping into privacy issues.
Looking Ahead
Finkenstadt: I don’t want to suggest that we’ve cracked the code on everything. Instead, we cracked the code on concerns. If you’re thinking, “I haven’t really seen the details of the response to this and how it’s going to work,” that may be because they don’t exist. You need to start putting your heads together.
Handfield: One of the important things in any supply chain plan is awareness — awareness of all the possible things that could go wrong. If you’re working in an organization, you need to consider the risks that we’ve identified here. We would urge policy makers at a state and local level to start thinking about these logistics issues and how to deal with these high-touch complex handling procedures.
This post was originally published in Poole Thought Leadership.