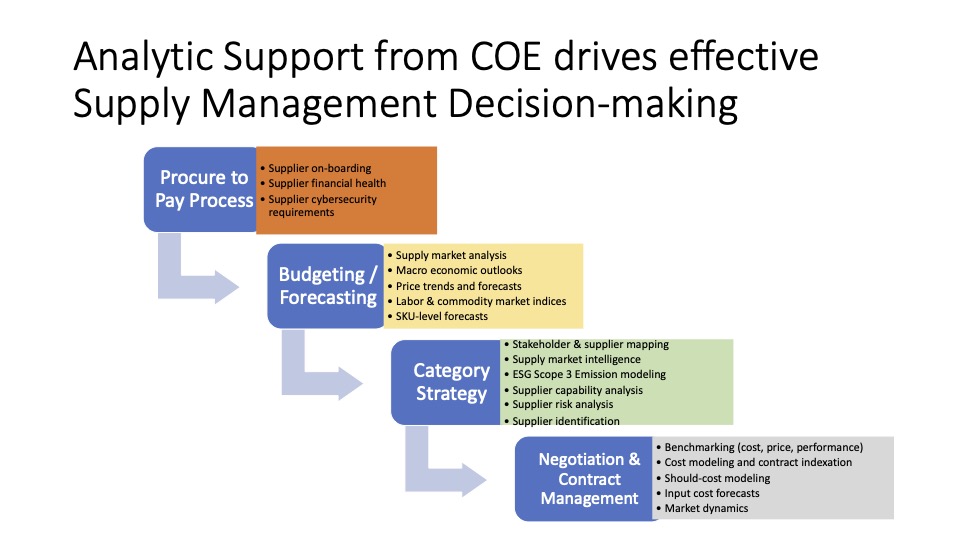
Problems Facing the New Pharmaceutical Industry – Part 2
No one would disagree that new products are the lifeblood of the pharmaceutical and biotech industries. Almost every company in this sector is focused on developing new products, grow revenues, and reducing the cost of doing so, which together will add up to a positive return on assets, which in turn will help drive the company’s stock price up and satisfy stockholders, all during a very challenging economic environment. However, every business strategy, no matter how visionary, ultimately boils down to hard work. In most cases, this means specific team-based projects focused on improving existing processes to achieve these goals. The sum of these project results is ultimately how we measure the success of a strategy. One of the strategies that many pharmaceutical and biotech companies are investigating today is to outsource many of the traditional “non-core” elements of their business. This means first defining what IS core to the business. To quote Martin Steinman, Schering-Plougd Research Institute:
“Our core competency is development of new drugs – bringing them from discovery to the marketplace. Thus we are amenable to outsourcing of intermediates. In general we want to do final steps in-house, especially to control the GMP steps.”
Outsourcing is certainly an attractive option – so long as you remain world class in the PROCESS of outsourcing, and can devote resources to doing so. Many executives forget that outsourcing isn’t something that eliminates current deficiencies in your business processes. In fact, outsourcing bring with it a whole set of new challenges, and requires a maturity procurement organization to MANAGE the outsourcing arrangement. Outsourcing can provide benefits when an outsourcer is able to control the product, the process, the documentation, and the quality systems. However, this cannot occur without the sponsor actively “managing” the service provider as well, through detailed metrics and audits as required before, during, and after a project. If the technology is well defined and the service provider has significant experience with it, these audits may be less intense in nature. However, in a not fully mature technology or one that the service provider is not familiar with, the rate of audits and measurement should be intensified. One of the greatest predicted success factors discovered in an NSF sponsored research study of supplier integration in new product development, is that a prior experience with a service provider is likely to be a high predictor of future success(1).
In response to this trend towards outsourcing, a whole new generation of companies known as Contract Research Organizations (CRO’s) are filling the void. CRO’s are increasingly becoming responsible for a broader breadth of services – especially with the integration of companies such as Magellan’s recent acquisition by Cardinal Health (link to our project) to create comprehensive pharmaceutical development service provider to the pharmaceutical and biotechnology industries, from early stage drug development to commercial manufacturing and distribution for virtually every dosage form.
As companies move into co-development, co-promotion, and co-marketing arrangements, the degree of invasiveness reaches epic proportions. These agreements are often structured based on complete and total joint government, implying consensus building at every level. Unfortunately, the mechanics of making this happen are difficult to operationalize at a detailed functional level. Moreover, both parties require extensive relationship management skills, particularly in the area of procurement, contracting, and strategic alliances. These types of skills can be acquired intensive training, through role plays, simulations, and face-to-face interaction.
Reference:
(1) Handfield, Robert B. and Ernest L. Nichols, Jr., Supply Chain Redesign: Transforming Supply Chains Into Integrated Value Systems, Prentice Hall, Inc., Upper Saddle River, New Jersey (2002) (ISBN: 0-13-060312-0).
- Categories: